Thousands of children are born with profound hearing loss, Otolaryngology Chair Emeritus Jonas Johnson said at the beginning of the Eye & Ear Foundation’s August 24th webinar entitled, “Hereditary Hearing Loss: New Innovations for Treatment.” The good news is they can receive cochlear implants. The better news is that modern science is showing that more can potentially be done.
Normal Hearing
Dr. David Chi, Associate Professor of Otolaryngology at the University of Pittsburgh School of Medicine, explained how normal hearing works. He serves as the Hearing Center and Cochlear Implant Program Director at Children’s Hospital of Pittsburgh, is on the State of Pennsylvania Department of Health Hearing Advisory Committee and is an active member of the Infant Hearing Screening Committee.
Sound causes the eardrum to vibrate and move. When this occurs, three bones in the middle ear also move. This causes audio to have a fluid shift in the inner ear, which causes the displacement of hair cells (one row of inner hair cells, and three rows of outer hair cells). This hair cell movement creates a release of neurotransmitters, which causes electrical signals to traverse through the hearing nerve to the brain. We perceive this as sound.
Damage to these hair cells is thought to be the cause of hearing loss in children and adults.
Incidence of Hearing Loss
Did you know that hearing loss is the most frequent birth condition? Approximately 12,000 infants are born annually in the U.S. with hearing loss, which correlates to about 35 babies a day.
Hence, early detection and treatment of sensorineural (hearing loss in the inner ear) hearing loss is vital. Dr. Chi called it a neurodevelopmental emergency, because early intervention has significant benefits, including better speech and language scores.
PA and almost all other states have universal newborn hearing screening. In these states, the goal is 1-3-6. In other words, screen by one-month, audiological diagnosis by three months, and early intervention by six months.
In 2019, the Joint Commission of Infant Hearing established new guidelines: 1-2-3. Screen by one month, diagnosis by two, and intervention by three.
Causes of Hearing Loss
What causes hearing loss in children? Twenty-five percent acquire it, 25 percent is unknown, and 50 percent is genetic.
One third of hearing loss is syndromic, while two thirds is non-syndromic. There are more than 500 syndromes.
Of the non-syndromic hearing loss, 60-80 percent is autosomal recessive, 15-20 percent is autosomal dominant, 2-3 percent is x-linked, and less than five percent is mitochondrial.
Autosomal Dominant
- One parent usually has hearing loss
- Parent with 50 percent risk of transmitting to child
Autosomal Recessive
- Require inheritance from each parent
- Parent usually does not have hearing loss – parents are carriers
- 25 percent of these parents’ offspring will be affected
Connexin 26
- DFNB1 mapped to chromosome 13
- Mutation of gene GJB2
- Mutation associated with 50 percent of autosomal recessive hearing loss
- Carrier rate in Midwest US: 2.5 percent
Connexin is a “docking station” for cells to communicate with each other. Connexin proteins are present throughout the inner ear structures. Mutations in this protein lead to cellular dysfunction and deafness.
Acquired Hearing Loss
- Infection
- Ototoxins
- Prematurity
- Kernicterus (severe jaundice)
- Birth anoxia
Prematurity
- Most significant perinatal factor
- 20-fold increase in hearing loss
- 5 percent of NICU grads have hearing loss
What is Cytomegalovirus?
Dr. Chi referred to cytomegalovirus as another dreaded virus that starts with “C.” It is a herpesvirus that causes $4 billion/year in medical expenditure. It affects 0.5 to one percent of all newborns, which equates to 30,000 congenital CMV infections. It is the most common cause of nonhereditary SNHL, account for 20-25 percent of early childhood hearing loss.
CMV: Symptomatic Congenital Infection
- 5-10 percent
- Prematurity
- Common features:
- Enlarged liver
- Enlarged spleen
- Low platelets
- Jaundice
- Microcephaly
- Sensorineural hearing loss (50 percent)
CMV: Asymptomatic Congenital Infection
- 90-95 percent or more of these infants have no sequelae
- 5-15 percent have SHL
- Hearing loss can be evident at birth or appear later in childhood
- No predictable method to which children get hearing loss
Degrees of Hearing Loss
Hearing loss may range from mild to profound. In this case, hearing aids are the primary option. If a severe to profound loss, children may not benefit from hearing aids alone.
Hearing Aids vs Cochlear Implants
Hearing aids amplify sound. For a person with severe to profound loss, a hearing aid does not provide sufficient clarity for understanding of speech. In this case, a cochlear implant may be beneficial, as it bypasses the damaged part of the ear.
Cutting Edge Research
Dr. Christopher Cunningham is an Assistant Professor in the Department of Otolaryngology and the Pittsburgh Hearing Research Center at the University of Pittsburgh. The Cunningham Lab is interested in understanding the neural and sensory biology of the vertebrate auditory system. Basically, his work is to treat the underlying cause of hearing loss.

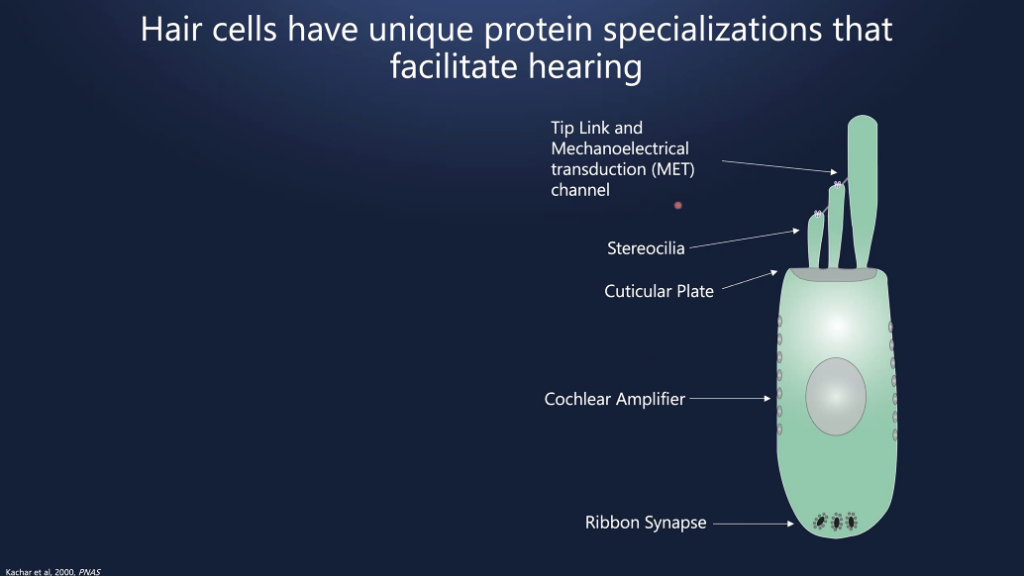
Dr. Cunningham said hearing loss is the most common sensory deficit. One in eight people in the U.S. aged 12 or older has hearing loss in both ears. The most common cause of sensorineural hearing loss is dysfunction of hair cells in the cochlea. The hair cells in the cochlea are the mechanical sensors of the auditory system and have unique protein specializations that facilitate hearing. Hearing loss is often due to improper localization of critical proteins.
DNA provides instructions to make proteins. But when there are DNA mutations, that leads to dysfunctional proteins in the cochlea, which results in hearing loss. Hundreds of different gene mutations cause hearing loss.
Summary of genes identified to date:
- Total non-syndromic HL genes identified to date: 124
- Autosomal dominant non-syndromic HL genes: 51
- Autosomal recessive non-syndromic HL genes: 78
- X-linked non-syndromic HL genes: 5
There are 700 different syndromic forms associated with hearing loss.
Because Dr. Cunningham wants to understand hearing loss better and develop therapies, he uses the mouse as a model for studying genetic hearing loss. The cochlea anatomy in humans and mice is very similar. Additionally, we have the same proteins, perceive sound the same, and nearly all genes critical for non-syndromic forms have been studied in mouse models. Most hearing loss in both has the same pattern. So, in mice, we have a powerful model to study things with direct implications for human hearing.
“Studying deafness-linked genes in mouse models provides insights into mechanisms of auditory function and novel therapeutic targets,” Dr. Cunningham said.
How Hearing Loss is Studied in Mice
- Mouse genetics to generate same DNA mutations in mice as in humans
- Hearing function testing using Auditory Brainstem Response (ABR) and other tests
- Microscopy to look at molecules, cells, and structures of the cochlea
- Biochemistry and molecular biology to test how deafness-linked mutation affect structure and function of specific genes and proteins
- Therapeutic development to generate targeted hearing loss therapies in mice with the goal for human patients
Humans and mice with genetic mutations in LRTOMT/TOMT have profound deafness. Hearing in mice is measured with an ABR. Little electrodes are placed under the skin near the ear. Sounds are played at varying intensities and pitches/frequencies. Neural activity is then recorded, so scientists can figure out what intensity and frequency of sound results in particular neural activities. “This is essentially telling us how well the mouse hears,” Dr. Cunningham explained.
With the TOMT gene mutation that causes profound deafness, sound waves are not sensed properly in the cochlea. This is because the TOMT gene is essential for proper MET channel localization. The goal then is to see whether therapies can cause the channel to return to the correct location.
Gene Therapy in Hearing Loss
Current therapies for hearing loss are hearing aids or cochlear implants. No biological therapies exist. Treatment basically is an attempt to minimize/overcome secondary effects of hearing loss.
With gene therapy, the goal is to directly address the primary cause of hearing loss (DNA mutations). Multiple biotech companies are investing a lot in gene therapy for different forms of hearing loss. Over 100 different gene mutations cause hearing loss, at least for non-syndromic hearing loss.
Delivering deafness-linked genes to the hair cells of the cochlea involves injecting adeno-associated viral vectors containing deafness genes into the inner ear. After a period of time, a microscopic analysis of the cochlear is done, followed by a hearing test. Did the gene of interest make it to the hearing cells? Was hearing function restored at all?
“We have optimized conditions so that gene delivery to cochlear hair cells is very efficient,” Dr. Cunningham said.
Gene therapy restores hearing function in mutant mice, he added. He shared some recent results that have yet to be fully analyzed. Hearing function was partially restored; but restoration right now is inconsistent. “We have some ideas (for how to improve the rescue),” he said. “We’re working to address this so we can get full hearing rescue across the entire cochlear axis.”
Restoring Vestibular Function
Often mice and humans with mutations in hair cell genes have balance issues. Gene therapy actually restores vestibular function in mutant mice. In fact, ALL treated TOMT mutant mice had normal (rescued) vestibular function.
Where We Are Now
- Efficient gene delivery to hair cells (but not 100 percent)
- Partial hearing rescue
- Vestibular/balance rescue
- Hints at TOMT mechanism (assembly/trafficking of MET channel)
- Promising gene therapy strategy
Dr. Cunningham said efficient gene delivery to hair cells is a pretty significant achievement. He thinks they will be able to get to 100 percent.
Next Steps
- Full hearing rescue (vector, conditions, timing)
- Therapeutic window (age of delivery)
- Precise function of TOMT
- Gene therapy with other deafness-linked genes
Dr. Cunningham is hoping to achieve full hearing rescue in the next year. “I think we can achieve it,” he said. “We have a number of ways to try to get there.”