The Eye & Ear Foundation’s November third webinar had a special guest: John Ash, PhD, the incoming E. Ronald Salvitti Professor of Ophthalmology Research, Vice-Chair, and Director of Research.
Alexander Anetakis, MD, MBA, Clinical Assistant Professor of Ophthalmology, Vitreoretinal Division, and a Senior Advisor to the Translational Sciences Team at UPMC Enterprises, was the other presenter. They both spoke about “Age-Related Macular Degeneration: Understanding Genetic and Behavioral Risk Factors to Develop New Therapies.”
Anatomy and Function of the Eye
Dr. Anetakis described the anatomy and function of the eye. He explained that macular degeneration is a disease of the entire retina but primarily affects the macular, a critical structure. It is involved with reading, driving, recognizing a face, and watching a ball game. All the important things we think about vision come from the macula.
Age-Related Macular Degeneration
This disease has a large patient population size.
- Over 10 million patients in USA
- Affects 1 in 8 people 60 years of age or older
- Most common cause of irreversible blindness in older persons in developed countries
- 200 million people worldwide
- Projected 288 million people by 2040
What do these patients see? They can lose most of their center vision. Even the peripheral vision is somewhat affected.
Current Thoughts on Mechanism of Action
“I want patients to understand how complex of a disease this is,” Dr. Anetakis said, “and why it’s so hard to attack this problem. If you hit any one part of the pathway, there’s other ways for the pathway to get around. It’s such a challenging disease to really attack and try to find a solution for.”
Non-Exudative ARMD Current Treatment
These were very large studies with strong scientific proof that these vitamins really are effective, but unfortunately the efficacy was not as robust as hoped, and the results from AREDS2 were not superior to the original formula, Dr. Anetakis said. They do slow the disease, and they are still worth taking.
- AREDS Vitamins – two formulae (AREDS formula and AREDS2 formula)
- AREDS 1
- Results in 2001
- 3,640 patients
- Reduced chance of progression to advanced ARMD by about 25%
- AREDS 2
- Results in 2006
- 4,203 patients
- Similar results as AREDS 1
- AREDS 1
There is ongoing innovation in the retina pipeline, with research, clinical trials, and different ways different companies are attacking this problem. The most common or robust is looking at inflammation – ways of modulating it.
Non-Exudative ARMD Phase III Therapeutics
- APL-2 C3 Inhibition (Apellis)
- Monthly or every other month intravitreal injections
- Phase III trials
- OAKS
- Reduction in geographic atrophy (GA) growth by 22%/16%
- DERBY
- Reduction in GA growth by 12%/11%
- Did not meet primary endpoint
- OAKS
- Phase II Trial
- Filly
- Reduction in GA growth by 29%/20%
- Filly
- Exudative AMD Incidence
- 6.0%/4.1% experimental
- 2.4% sham
- Apellis has PDUFA date with FDA on 11/26/22
Zimura C5 Inhibition (IvericBio)
- Monthly intravitreal injections, 2 mg or 4 mg
- Phase III trials
- Reduction in GA growth by 27.4%/27.8%
- Exudative AMD Incidence
- 9.0%/9.6% experimental
- 2.7% sham
- Iveric Bio filing New Drug Application with FDA by end of Q1 of 2023
Exudative ARMD
Large Patient Population
- 15% of ARMD patients
- Approximately 1.5 million patients in the US
- Approximately 30 million patients worldwide
Untreated, it leads to legal blindness within two years in 95% of patients.
Current Treatment
- Anti-Vascular Endothelial Growth Factor Intravitreal Injections
- Bevacizumab (Avastin)
- First used in 2004
- Off-label use of drug
- Ranibizumab (Lucentis)
- Approved in 2006
- Aflibercept (Eylea)
- Approved in 2012
- Brolucizumab (Beovu)
- Approved in 2020
- Faricimab (Vabysmo)
- Approved in 2022
Exudative ARMD Current Treatment (non-intravitreal injections)
Susvimo
- Ranibizumab depot device
- Refillable every 6 months
- Approved in 2021
- Recalled 2022
- Photodynamic therapy
- Visudyne intravenous injection combined with laser therapy
Exudative ARMD Phase II and III Trials
Long-Acting anti-VEGF intravitreal injection
- KSI-301 (Kodiak)
- Phase III trials
- Initial results similar to faricimab
- Sustained drug delivery platforms
- OTX-TKI (Ocular Therapeutics)
- Phase II trials
- Preliminary results promising, up to six months in 50% of patients
- GB-102 (Graybug)
- Phase II Trials
- Preliminary results promising, up to six months in 48% of patients
- Some safety concerns
Gene Therapy anti-VEGF
- RGX-314 (Regenxbio)
- Suprachoroidal injection in Phase II trials
- Preliminary results show reduction in rescue dosing of 75.9%
- ADVM-022 (Adverum)
- Intravitreal injection in phase I/II Trials
- Preliminary results show reduction in annualized by 81-98%
Conclusions
- ARMD is a significant vision threatening disease in the USA and worldwide
- However, most patients can maintain some functional vision
- Significant advances in the past 15 years have provided significant hope
- Future advances expected to lower impact on patients’ vision and quality of life
- Still significant research and development needed
Dr. Ash Joining the Department
Dr. Ash’s official start is December 1, and he is very excited. There are 22 research faculty in the department. Most of them are fairly junior, at the cutting edge of their research fields and the most creative point of their career. “It is exciting to be able to help establish a culture in the department,” Dr. Ash said, “so that we can enable faculty to do their best research and move the field forward.”
Dr. Ash gave a shout out to the “fantastic partnerships” with the University of Pittsburgh Ophthalmology Department and the Eye & Ear Foundation. The partnership promotes research projects and opportunities to enable translational development. The end goal of any scientific creation or invention, after all, is to benefit the patients. “There is a huge gap between discovery and getting new therapies to patients,” Dr. Ash said. “Infrastructure at bridges over the gap to promote therapy development and commercialization.”
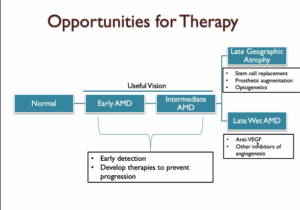
AMD is progressive with various stages of vision loss. Patients in the early stages of AMD still retain significant useful vision, while patients with late-stage disease have substantial vision loss. Dr. Ash’s research focuses on developing therapies for early and intermediate stage patients in order to stop progression to later stages. As a researcher, he believes patients would have the best outcomes if we could develop therapies to maintain useful vision for longer. However, Dr. Ash is also excited about therapies on the horizon for patients who have already lost useful sight.
New Therapies
New therapies are under development that affect metabolism:
- Drugs (Metformin, 8-OH-DPAT)
- Proteins (LIF, OSM)
- Gene Therapies (LIF, STAT3, PGC-1a)
Some are FDA approved. Metformin has had the most work and can be effective, but new drug development is needed.
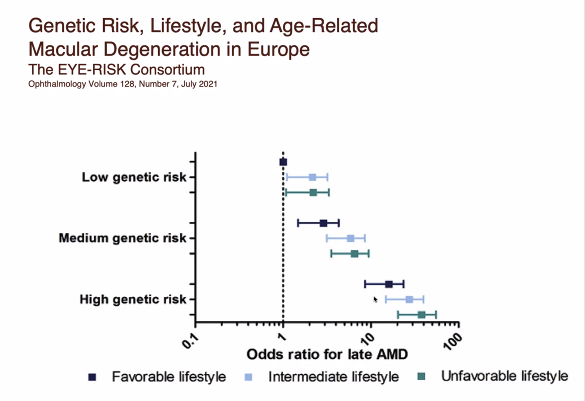
Dr. Ash said that the overall risk of developing AMD is a combination of genetics and behavior. He reported that recent studies have identified changes in 32 different genes that are linked to developing AMD, and that it was possible that people can inherit multiple DNA changes from their parents. AMD Is a complex disease, and the risk of developing AMD is determined by the number and types of DNA changes inherited. Combinations of DNA changes can put patients at higher risk of disease. However, simply inheriting the DNA changes is not enough to cause AMD, which is the major reason why genetic testing is not recommended for diagnosing or predicting AMD. Dr. Ash mentioned that patients with high-risk genetics can reduce their chances of developing AMD by adopting lower-risk behavior.
How to Lower Risk
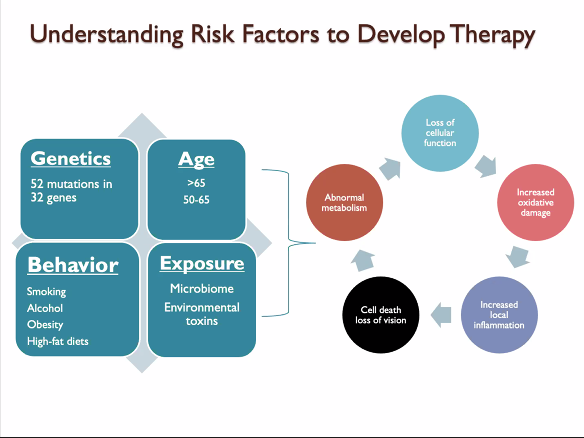
- Avoid high-fat and high-glycemic-index diets
- Don’t smoke
- Take AREDS2 supplements
- Moderate alcohol consumption
- Regular exercise and reduction of system inflammation