The FDA finally approved over the counter (OTC) hearing aids. Now what?
For the last three years, Catherine Palmer, PhD, Professor and Interim Chair in the Department of Communication Science and Disorders, Professor in the Department of Otolaryngology at the University of Pittsburgh School of Medicine, and Director of Audiology for the UPMC Integrated Health System, has been working with the American Academy of Audiology as Chair of the task force related to OTC hearing aids to help audiologists get ready for the new reality.
She imparted her knowledge at the Eye & Ear Foundation’s November 15th webinar, entitled, “Everything You Need to Know About OTC Hearing Aids.”
History
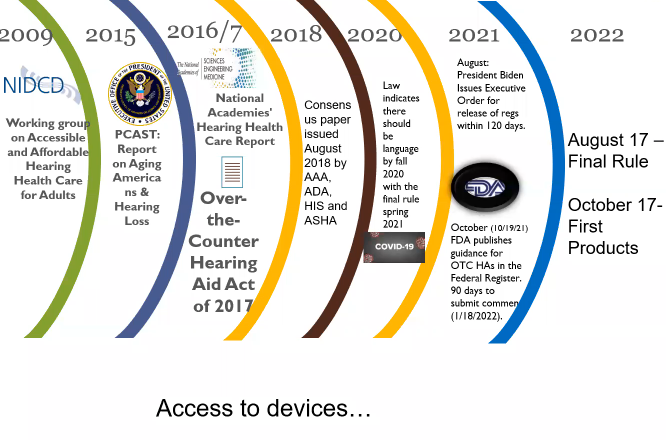
This FDA rule has been a long time coming. The goal of the final rule is to make access to amplification devices more affordable and accessible in terms of eliminating the need for appointments and professional assistance. The majority of aging adults, if they live long enough, will have some level of hearing loss. This is considered a public health issue.
Senators Chuck Grassley and Elizabeth Warren – who do not often work together – came together and created the OTC Act of 2017, requesting that within three years the FDA come up with a category of devices that would be direct to consumer to potentially help with hearing. A lot happened in 2020, so the original draft was not published until 2021.
When a draft rule is published, anyone can submit comments. The FDA received about 937 comments, which they responded to in a formal way. The final rule came out August 17, 2022, with the first products on the market October 17, 2022.
Access to Devices, Not Services
Dr. Palmer pointed out that what we do not have is health insurance that covers both devices and services needed for people to get maximum benefit from hearing aids and other assistive listening technologies. There are a couple of bills that are in process, trying to get Medicare to cover hearing aids and services, for example. Dr. Palmer said to keep an eye on this and think about it when you vote if you consider this an important issue.
OTC Hearing Aids
- Increase access by creating a self-care pathway (but if this doesn’t do what you need it to do, or if you end up having pain or any other kind of problems, then you would go to an audiologist or ENT specialist to get care)
- For adults with perceived mild to moderate hearing loss (“The problem potentially with that is that we know from years and years of research that individuals don’t accurately identify the level of hearing loss they have,” Dr. Palmer said. “The best advice would be to get a hearing test, so you know what level of hearing loss you have.”)
- Available for use by consumers without professional assistance
- Lack of constraints
- “No State or local government shall establish or continue in effect any law, regulation, order, or other requirement specifically related to hearing products that would restrict or interfere with the servicing, marketing, sale, dispensing, use, customer support, or distribution of OTC HAs.”
The rules come from the FDA, so they are preempting state rules that have dictated more traditional hearing aids, Dr. Palmer explained. To be clear, they are not changing the rules for traditional (now called “prescription”) hearing aids. They are saying that states can dictate the more traditional hearing aids that are dispensed by audiologists and fit by them, but they can’t constrain the OTC hearing aids that people can get directly. The state cannot put on extra requirements. The whole point of the FDA rule is to get rid of those requirements.
Prescription vs OTC Hearing Aids
When an audiologist programs hearing aids specifically for the individual, they’re now calling this a prescription hearing aid. The FDA defines “Prescription Hearing Aids” as those subject to the requirements in 801.109, including that “they be sold only to or on the prescription or other order of a practitioner licensed by law to use or order the use of or prescribe.”
The FDA defines OTC hearing aids as “devices available OTC without the supervision, prescription, or other order, involvement or intervention of a licensed person, to consumers through in-person transactions, by mail or online.”
Other Categories of Devices
Many people ask about the difference between devices. The biggest difference, Dr. Palmer said, is the intended use and guardrails that the manufacturer puts on the device.
- Personal Sound Amplifier Products (PSAP) (These are not allowed to be advertised for helping with hearing loss and are meant to enhance hearing in certain situations.)
- Hearable (like wireless earbuds or headsets that let people hear things not necessarily tuned a certain way for hearing loss but with volume control)
- OTC hearing aid
- Prescription hearing aid
Output Limiting
- 111 dB SPL
- 117 dB SPL when there is input compression in the signal processing
The FDA first proposed that these devices could be up to 120 dB, which is excessively loud and would be damaging to hearing if you really exposed yourself to that even for a short period of time, Dr. Palmer said. So, they lowered it. Certain combinations could go up to 117.
“Both levels could injure hearing if someone listened at this level for a long time – but this is the maximum,” said Dr. Palmer. “We don’t expect people to wear it at this level, but there’s nothing stopping them from that. People would want to be thoughtful with how loud they’re wearing the OTC hearing aid.”
Dr. Palmer put a plug in for the Audiology Clinic. “If someone buys an OTC hearing aid, and they’re not sure about how they set it or how loud it really is, they can come into an audiologist,” she said. “We can measure it because we can measure anything that makes sound. We can also give them some advice about appropriate use.”
Returns
Something to keep in mind is that the FDA is not requiring OTC hearing aid manufacturers to allow returns. The return policy should be clearly stated on the outside of the box.
“I would strongly recommend that if you or a friend are thinking about this, that you purchase a device that can be returned,” Dr. Palmer said. You have no idea how it will work or if it will even help until you try it.
Note: OTC hearing aids are not going to be inexpensive. It will likely be $300-500 per device, and if there is hearing loss in both ears, two should be worn.
In Southwestern Pennsylvania, no one needs to go without hearing aids, Dr. Palmer said. The Eye & Ear Foundation helps support three free hearing aid clinics, in addition to the annual Mission of Mercy Pittsburgh free hearing aid clinic (the next one is June 23-24, 2023).
Fitting Devices
Notably, the FDA is silent on how the devices are fit. They call this customization, but Dr. Palmer thinks a better word is adjustment. There are two adjustments that must be available on OTC hearing aids: pitch and loudness.
Distributor Requirements
The FDA is not requiring any kind of 800 or other phone number to be in touch with the manufacturer if the OTC hearing aid user needs assistance. Distributors, however, are required to provide an e-contact along with website information. The new FDA rule applies only to those who are 18 and older; the rule specifically indicates that OTC hearing aids are not for individuals under 18. In PA, children up to the age of 21 receive hearing aids at no cost regardless of income. This is not true in any other state.
Hearing Loss Demographics
When thinking about people with hearing loss, there are two groups: one that recognizes they have hearing loss, and one that doesn’t realize they do. Of the people who recognize they have hearing loss, there is a split. Audiology clinics typically see the 8.7 million people who are ready to do something about their hearing loss. About 3.4 million do not pursue care for a variety of reasons.
The availability of OTC hearing aids may impact these 3.4 million people. Perhaps they will be more willing to get hearing aids if they are a little less expensive and do not require going to a clinic.
Interestingly, 26 million people with hearing loss don’t realize they have hearing loss. It would be ideal if people over 60 years of age had a baseline hearing test so hearing loss could be identified and treated sooner rather than later.
Individualized Hearing Aid Fittings
Audiologists customize the physical and acoustic fit of a hearing aid so it matches the person’s needs, ear canal, and hearing loss. Audiologists work for about an hour to fit hearing aids, but the person wearing them must work for about three weeks to adapt to these new sounds.
People with hearing loss have been walking around with the hearing loss for a long time before deciding to do something about it. The average length of time is seven years, according to Dr. Palmer. Maybe it will be shorter with OTC hearing aids.
The hearing loss acts as a filter and the brain is tuned to hearing through that hearing loss. The brain thinks it is normal. “We always say to patients, if we do a good job fitting hearing aids and bringing that sound back, especially those high pitches, it will not sound normal in the beginning, because the brain is not used to it,” Dr. Palmer said. “It’s going to sound sharp and tinny, too loud. The key is to wear it all your waking hours to allow your brain to adapt.”
The first week will not sound pleasant because the brain pays attention to everything and cannot tune out unwanted sounds. Repeated exposure helps.
People are not good at self-tuning amplification because they tend to want to change the sound when it does not sound good rather than let their brain adapt to the new sounds. This is potentially a problem with OTC hearing aids because consumers will have access to the controls. They may not have the aid at a level to provide maximum benefit.
Studies have also found that most people do need assistance with devices. Just opening a box and figuring it out is not easy.
Data indicate that if you try to wear a hearing aid part-time, you will never do as well as someone who wears them full time. “That’s because you keep your brain in adaptation mode,” Dr. Palmer said. The expectation with OTC hearing aids is that people who pursue them will be more part-time users.
Best Advice
Dr. Palmer’s parting words of advice are to get a hearing test (this should be covered by insurance) and to get recommendations based on the hearing results and your goals. Coming in for a hearing test and talking through things with an audiologist will save time and money in the long run.