Kevin J. Contrera, MD, MPH, Assistant Professor, Department of Otolaryngology-Head & Neck Surgery, opened the Eye & Ear Foundation’s May 6 webinar, “Personalizing the Treatment of Head and Neck Cancer,” by saying that this is the focus of his work. Sometimes it is called precision oncology.
“While there are a lot of ways to think about it, in its simplest form, it is about customizing the cancer treatment to the patient at hand,” he added. Sometimes this is done based on the needs and desires of the patient, but many times it is based on the tumor’s biology. This kind of treatment is done through clinical trials.
Changing the Paradigm
Traditional treatment is surgery followed by risk-based radiation and chemotherapy. The thinking is to do surgery first and ask questions later; the type of tumor does not necessarily matter. Based on the results, the patient would then have radiation or radiation and chemotherapy. It is a cookie cutter approach where the tumor is treated based on where it is and where it is not, but there is not a lot of further precision beyond that.
Over the past decade, there have been a lot of advances within the therapeutic space, with novel things like immunotherapy and targeted therapy. It can mean just taking a pill or sometimes getting drugs through an IV. “As surgeons, we are responsible for integrating these exciting new advances into the treatment of head and neck cancer (HNC),” Dr. Contrera said.
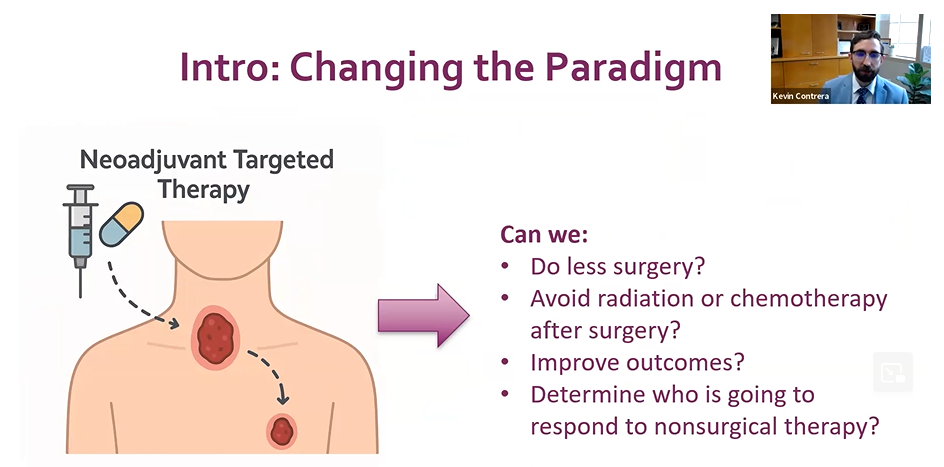
One of the major ways Dr. Contrera is working on that is through something called neoadjuvant targeted therapy, where a patient is given a drug prior to surgery. The tumor subsequently responds to that and, in many cases, shrinks. That allows the surgeon to adapt and customize the subsequent surgery.
Exploring this therapy means answering questions like, can we do less surgery, avoid radiation or chemotherapy after surgery, improve outcomes, or determine who is going to respond to nonsurgical therapy?
Even though HNC is the sixth most common cancer worldwide, most people have probably never heard of it. This is because it is made up of a lot of different types of cancers, which include thyroid, nasal cavity and sinuses (sinonasal), skin, and mouth and throat. Each of these cancers is kind of its own category and is treated very differently.
Neoadjuvant Selective RET Inhibitor for Medullary Thyroid Cancer
One of the worst outcome thyroid cancers is medullary. In 2020, the FDA approved a targeted therapy called selpercatinib for thyroid cancers with mutations for the gene RET. A Phase II trial is ongoing for patients with advanced medullary thyroid cancer with RET inhibitor therapy followed by surgery. Selpercatinib is then restarted after surgery based on biomarkers. Dr. Contrera presented the first series of patients treated this way.
Given how aggressive this type of cancer is, the results were quite notable. Tumors shrank on average by 40%. An estimated five speaking nerves were at risk before neoadjuvant therapy, but only one nerve was ultimately sacrificed in surgery. All patients are alive at 2.0 years without structural disease.
“Medullary thyroid cancer is unique in terms of HNC because there are known biomarkers, which are blood tests that track how the cancer is doing,” Dr. Contrera said. It is not a standard one-size-fits-all approach, but once levels have plateaued, they proceeded to do surgery to take out all the remaining cancer they could see. The assumption is there are probably some cancer cells left because the markers of disease have ticked back up. The drug was then restarted, and the numbers went back down to nearly undetectable.
Frequency of Targetable Mutations/Fusions
Thyroid cancer has come a long way, where it is no longer just about what type of cancer you have, but what the genetic mutations are that drive a cancer. In some cases, the mutations matter the most because there is a cocktail of specific drugs that can be used to target depending on the mutation. Although not the standard approach, this is very much the direction where things are going.
Induction Chemotherapy for Advanced Sinonasal Squamous Cell Carcinoma
This is a much rarer cancer. It is harder because it is hidden within the nose. Traditionally it is very aggressive and tends to have poor outcomes. A Phase II trial evaluated previously untreated patients with stage II-IV treated with induction chemotherapy, meaning it was given first instead of after surgery. All patients would get chemotherapy if their tumors did not shrink significantly with chemotherapy. In that case, they know surgery is likely the best tool for getting it all out. If the patient had a good response to chemotherapy, they got one more dose, followed by radiation and chemotherapy. They only had surgery if there was still some cancer remaining.
In 28 patients, nearly all had advanced/stage 4 disease. Eighty percent of patients had their tumors shrink because of neoadjuvant therapy. At two years, more than 2/3 of patients were alive. At five years, just under half of the patients had no evidence of disease. “Obviously we hope this could be better,” Dr. Contrera said. But looking at past numbers, these are substantially better.
Most notably, patients would have required maxillectomy (89%), craniotomy (54%), or removal of eye (29%) with the standard upfront surgical approach. In the trial, two-thirds of patients had additional organ preservation, including avoidance of maxillectomy (face bone removal) in 38%, craniotomy (entering brain space) in 13%, and orbital exenteration (eye removal) in 38%.
Skin (Cutaneous) Cancer
In a trial with neoadjuvant cemiplimab and surgery for cutaneous squamous cell carcinoma, 79 patients had this kind of advanced skin cancer. They received up to four doses of immunotherapy followed by surgery. Half of patients had no live cancer at the time of surgery. Forty patients had their tumors removed surgically, and an additional 10% of patients saw major improvement.
In this trial, the vast majority of patients had very significant tumor shrinkage. Although Dr. Contrera did not have a role in the study, he is now at Pitt working with Dr. Neil Gross, senior author on the study, to pool their data since there are less than 80 patients. To get really good data on how patients are doing long-term, they have to work with other centers. They are sharing data across the entire globe, including Australia and Italy.
“The only definitive way of answering the question, does this approach help patients live longer and do better, is through a randomized trial,” Dr. Contrera said. This means patients are selected not by their surgeons, but by the study on whether they end up getting treated with the standard approach – upfront surgery followed by risk-based adjuvant therapy, meaning radiation or chemotherapy – or the novel treatment, where they get neoadjuvant immunotherapy followed by surgery and radiation as needed. This is a cooperative national group trial that just opened, which Dr. Contrera is helping lead. In fact, Pitt has enrolled some of the first patients treated in the trial.
In this experimental arm of the study, surgeries are done on all patients, but they are response adapted surgeries. If a tumor has shrunk, historically, they always had the same surgery that would have been done prior to shrinkage. For this study, surgeons are permitted to reduce the margins they resect, so they are able to do a much smaller surgery. If they find out after tissue analysis that there is no viable cancer left, then for the first time, people will avoid radiation entirely. This can only be done in the clinical trial.
Mouth and Throat (Mucosal) Cancer
This is cancer of the linings of the airways and digestive tract. In a trial for neoadjuvant immunotherapy for mucosal squamous cell carcinoma, 42 patients were treated with one of three therapies prior to surgery: nivolumab, nivolumab + ipilimumab, or nivolumab + relatlimab.
These drugs are different ways of using immunotherapy to improve outcomes. They are done upfront and followed by surgery in all cases but by randomizing patients to each of these arms, physicians can get a better understanding of how the drugs are working. They found that compared to if only one type of immunotherapy was given, when two were given, patients with were 3-4 times more likely to have a major pathologic response. So far in these patients, cancers have not recurred and they are still alive and well. It is a very good sign of how they will do long-term.
Unanswered Questions
This is the research that still needs to be done, which Pitt is helping to lead. While these trials are very exciting, major questions are about response assessment: clinical, imaging, and biomarkers.
In a clinical response assessment, tools are patient-reported symptoms, physical exam, and biopsy. Limitations are that it is hard to see tumors, knowing what is cancer and what is not, and that biopsy can be unreliable.
Imaging has historically been the best way to assess response. When patients have chemotherapy, imaging is reliable. The difference with immunotherapy is quite striking; rather than just directly killing the cancer cells, the immune system is targeting the cancer cells. In some cases, it looks like the tumors are getting bigger because the tumors swell from inflammation, which is called pseudoprogression. Some of these patients progressed, but it is hard to tell which ones. Even though there is a lot of excitement about immunotherapy, it makes it harder to assess response, which is where biomarkers come in.
The goals of biomarkers are to predict response, assess disease burden, assess response, and prognosticate. Unfortunately, there are no standard biomarkers available for HNC squamous cell carcinoma. “That’s what our group has been focusing on,” Dr. Contrera said. “That’s been the sort of translational focus of a lot of my research, is can we use biomarkers to meet these goals?”
Biomarkers: Tissue-Based Biomarkers
CD8+ Tumor infiltrating lymphocytes are responsible for killing cancer cells and have shown to predict outcomes and response to treatment in various malignancies. HNC lags behind many other fields in the standardized use of this biomarker.
What they found when they looked at other studies investigating these biomarkers is that it does not matter the type of cancer. Tumors that have high levels of these have much better survival; in fact, patients are half as likely to die of cancer. “We can really start to have a big impact,” Dr. Contrera said. “One of the major ways that we can really move the needle is by being able to predict who is going to respond to which types of immunotherapy.”
In their study evaluating these different types of combinations of immunotherapy, they are learning how tumors look before and after treatment, and for the first time, they are able to assess and predict who might respond to which type of immunotherapy, which is groundbreaking.
Biomarkers: Blood-Based Biomarkers
During tumor destruction, tumor cells may release their genetic materials into the bloodstream, which is circulating tumor DNA (ctDNA). Dr. Contrera called this a very promising tool for prognosis and detection. He found that patients who had this ctDNA after all treatment was done were 7 times more likely to recur and 10 times more likely to pass away. Application of this has not been used readily in HNC, especially for tumors not caused by a virus. Looking to another disease site, specifically colorectal cancer, this was used to determine whether a patient was going to get adjuvant therapy. They found that when using this DNA, patients had comparable survival. But half as many patients needed chemo when they let the DNA determine rather than the standard pathologic analysis.
Now Pitt is working with the same company who did this trial with colorectal cancer, applying it to HNC in neoadjuvant period before surgery to get a better sense of figuring out how tumors respond. In turn, they can use that ctDNA to assess response. In theory, patients who have a good response could benefit from lesser treatment, while patients with a poor response may benefit from more aggressive treatment.
The bottom line: “There are a lot of opportunities to really personalize cancer care for HNC,” Dr. Contrera said.