At the start of the Eye & Ear Foundation’s August 22, 2025, webinar, “Translational Application of Artificial Intelligence in Head & Neck Cancer,” Steven B. Chinn, MD, MPH, FACS, thanked the Foundation for the invitation to talk about an area of technology near and dear to his heart. The Associate Professor, Co-Director of the Hillman Cancer Head and Neck Cancer Program, and Director of Research and Operations for the Computational Pathology & AI Center of Excellence (CPACE) at Pitt added, “We’re trying to drive integrating artificial intelligence (AI) into healthcare.” Just looking at the last two years of AI in terms of generative AI or image-making capacity, it has dramatically changed and shows what we are able to do.
Head and Neck Cancer (HNC)
HNC is categorized by the area in which the cancer arises:
- Upper Aerodigestive Tract Malignancy (mouth, throat, voice box, nasal cavity)
- Melanoma and Non-Melanoma Skin Cancer of Head and Neck
- Skull Base and Paranasal Sinus Malignancy
- Salivary Gland Malignancy
- Thyroid Cancer
The most common type arises from squamous cells, or the mucosal lining inside the mouth, nose, and throat. HNC makes up 3% of all cancers in the US, with ~63,000 new cases annually (46,290 men and 16,740 women) and ~13,360 deaths annually (9,940 men and 3,420 women).
Traditional Risk Factors
Cigarette smoking is implicated in 75% of all Head and Neck Squamous Cell Cancer (HNSCC) and means a 2-6x risk for a second HNC. Smokeless tobacco may result in HNC at the site of use and means a 4x risk in the U.S. Alcohol results in 3x risk with ethanol use alone. Betel quid, used in SE Asia and India, is another risk factor. Human Papillomavirus (HPV) is also on this list. Combined ethanol and tobacco use is a 10-15x increased risk.
There have been a lot of famous patients with HNC, like Rogert Ebert, Michael Douglas, Sammy Davis Jr., Sigmund Freud, Bruce Paltrow, and Gary Busey, among others.
HNSCC is a morbid disease with stagnant outcomes. In the last 75 years, Dr. Chinn said we have seen some improvement of survival. 1950-2000 saw improvement, but in 1990-2020, there has not been that much of an increase in the ability to treat this cancer.
“We can’t cure everyone, which is why we’re trying to figure out better ways,” Dr. Chinn said.
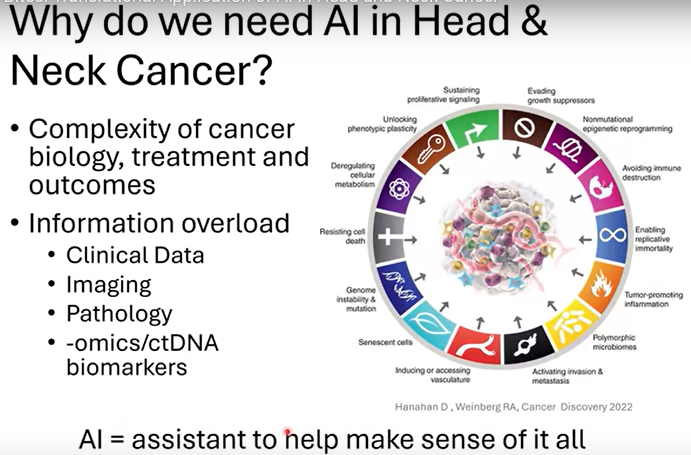
Why Do We Need AI in HNC?
There is a complexity of cancer biology, treatment, and outcomes. There is also information overload with clinical data, imaging, pathology, -omics/ctDNA biomarkers. An assistant, or AI, is needed to help make sense of it all.
If you open up any newspaper or magazine article, you will see something new with AI. This just reinforces how important this concept can be and why we really need to understand and harness it for good, “and in my case, for treating cancer patients,” Dr. Chinn said.
What is AI?
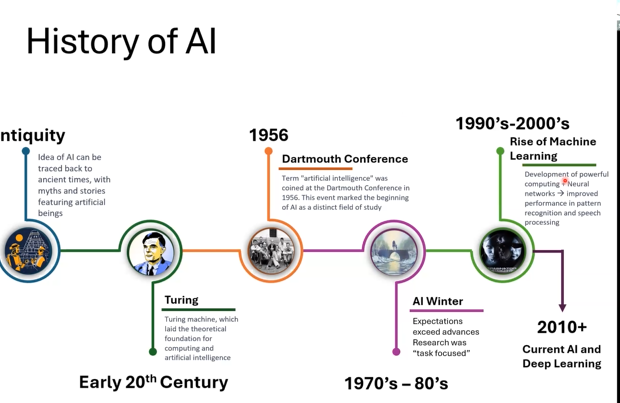
The Turing machine in the early 20th century laid the groundwork for AI. It was not until the late 2000s that we were able to harness our computational power. AI is a machine’s ability to perceive, synthesize, and infer information, and then perform cognitive functions associated with the human mind.
How does AI learn? Traditional programming, in which humans tell the computer exactly what to do, or machine learning, where the computer learns rules from data. In the latter, AI looks at lots of examples (like thousands of cat photos) and learns the patterns that define “cat.” Just like children learn by seeing, hearing, and practicing, AI learns from data and feedback.
AI is already impacting our lives. Just look at our use of smart phones, security and surveillance, social media platforms, navigation, e-commerce, banking and finance, autonomous vehicles, and smart homes. AI will not “take over,” but it will become part of nearly every field, making tasks faster and smarter.
Deep Learning
Deep learning is a subset of machine learning or teaching a computer model to learn by example – just like a child learns from parents and teachers.
Artificial neural networks (ANNs) extract abstract features from data and learn over time. ANNs need a much greater volume of data to train. Examples are driverless cars, Amazon Alexa, or Google Home. Take cars, for example. They look different and increasingly modern over the years. It takes time and data to train AI to identify something as a car even as it may not look like a traditional one. The quantity and quality of data matters.
AI and Medicine
AI has been integral in academic since the early 2000s. Back then, the vast majority of AI models were developed in academia. Over the last 20+ years, industry has taken over as leaders in terms of AI technology within healthcare. Roughly around 10% are still a combination of the two. “As a physician scientist, I have to be aware of where the technology is moving and who is driving it,” Dr. Chinn said.
Pitfalls of AI
- Requiring a large amount of data
- Data availability and quality
- Generalizability
- Technological limitations
- Black box/interpretability
- Scalability
- Most methods are tested in ‘simulated’ environments that cannot recapitulate real world clinical factors
- Ethical concerns
- Privacy
- Bias
- Negative impact on the doctor-patient relationship
- Regulatory issues
- Regulations are critical to guarantee the safety and efficacy of AI systems
- Takes time and effort
- Clinical trust
- Need for human-centered design
AI and Cancer
AI can help with screening, diagnosis, prognosis, treatment, surveillance, and survivorship. It has been very successful in identifying nodules based on a chest x-ray. A human would have looked at the x-ray and considered it normal, missing the centimeter nodule. AI models can be trained to pick up subtleties the human eye cannot necessarily pick up.
AI can predict treatment response based on imaging, determining how people are responding to the radiation therapy they are getting and assisting physicians in developing better plans to help patient care.
All the data that goes into the electronic model chart in theory is accessible to researchers and allows the data to be aggregated to start developing models to predict the outcome. These personalized risk scores can be used for symptom management and forecasting survival and recurrence probabilities. AI models also analyze clinical data to predict chemotherapy toxicity risk. Subtle symptoms can be flagged for early intervention.
-Omics
Some of the work Dr. Chinn did at the University of Michigan involved sequencing or looking at RNA and DNA within cancer. They were able to take an entire cancer and then deconstruct it using an AI model to determine what is in each cancer, the type of cells, and start to understand what makes up the cancer biology to help inform what is going on and how to better treat.
The University of Chicago was able to use some slides routine in cancer care to identify gene mutations. This typically involves a very costly and time intensive effort, but AI was able to do this just by looking at a picture of the cancer.
A study looked at using AI to drive adaptive radiotherapy by looking at the tumor to see if it was shrinking and developing a plan to optimize care. The study showed that AI was just as good as radiation oncologists at developing some of these plans. This was done in a very select group of patients but demonstrates some of the power of AI.
“The most exciting thing that we haven’t gotten too far into yet is something called Digital Twins,” Dr. Chinn said, “where I can take an individual patient and integrate all their data (clinical, x-rays, symptoms, etc.) and make a digital twin or a simulation of that person. We run different models, asking, if we do X, if we do Y, what do we think will happen? Will they respond better to this treatment? Or will they have a better side effect profile? This is where we’re starting to look at how we can integrate these digital twins into clinical trial development. Is it possible instead of enrolling 500 patients (which is costly and expensive) to use a smaller group and get the same answer?” It is still early, but this has high potential.
HNC AI Research at UPMC/Hillman
The Chinn Lab is developing AI models to improve diagnosis and prognosis of HNC.
- Digital pathology + deep learning -> biomarker discovery
- Integrating multi-omics data to understand tumor biology
- Predictive tools for personalized treatment and immunotherapy response
- Collaborative efforts
Immunotherapy is one of the newest kinds of therapies to combat cancer. It activates the immune system to fight cancer. Over the last 20+ years, there has been an explosion of immunotherapy trials for cancer. One of the challenges of HNC, however, is if it is given to everyone, only 20-30% actually have a good response. This means that the vast majority of people are not deriving benefit from immunotherapy.
Tumor Immune Microenvironment (TIME)
“So, what we believe is that HNC has a very specific makeup, with different cells, blood vessels, and organization,” said Dr. Chinn. “How do we untangle this to better understand why it does not respond to immunotherapy and why can we not improve those patient outcomes?”
In deep learning-based deconvolution, AI is used to deconstruct the cancers to better understand them. HNC is either an immune desert or wasteland, where there are not a lot of immune cells. Those are typically the ones that do not respond to immunotherapy. Other ones are hot and very responsive to immunotherapy. Using AI tools, researchers can start to better classify what they are trying to do from bench to bedside in terms of predicting the type of cancer and how to change a non-responsive tumor to a more favorable one that would respond to immunotherapy. This is called immune modulation or changing cancer’s environment.
Predictive Biomarkers
Current biomarkers miss the mark because immunotherapy is transformative but unpredictable. Only a subset of HNSCC patients benefit. What is in the tumor (TILs, PD-L1/CPS, TMB, CD8) is measured, but not where they are or how they interact; spatial context is ignored. Understanding this spatial aspect is really important and has not been fully looked at. The Chinn Lab is working on this.
Bench (Computer) to Bedside: Using AI to Deconstruct HNC
Employing digital pathology and convolutional neural networks for patient care and biologic understanding of cancer will lead to biomarker discovery and validation. The Chinn Lab is publishing and has a couple of grants.
Low levels of immune cells far away from the cancer did not do very well, for example, which tells us that the spatial aspect is important. AI is able to look at a standard slide that any lab can do and produce this result in under a second. “This is a very powerful tool for predicting how people will do with their therapy,” Dr. Chinn said.
AI-assisted mechanism discovery is integrating pathology with genomics and -omics to understand molecular components of the cancer.
Dr. Chinn talked about the concept of a black box. What they really need to know is what is happening. How did the computer look at the data and say who is going to do well and who will not? “Some of the work we’re doing is looking in that black box, opening it up, and starting to understand why cancers are behaving in certain ways, what is driving the neural network scores, something called Explainable AI (XAI),” he said.
What is driving the decisions that ultimately led to the output? By doing that, starting to identify interesting parts of cancer that may be driving whether the tumor is hot or cold, those areas can then be targeted to immune modulate and develop better therapies and make people more responsive to the drugs that exist or develop new ones.
Future Applications
Some of Dr. Chinn’s NIH grant work is in trying to identify pre-cancers. How can they take one and know it will convert into a cancer by looking at digital pathology? They are using different data points to plug into the AI model to develop the ability to pick out what it is. This is still very early in development, but Dr. Chinn believes it has very powerful implications in terms of cancer care.
AI in Cancer: Future Directions
- Early Detection & Screening: AI will enhance radiology and pathology workflows, enabling earlier identification of cancers through subtle imaging or tissue features invisible to the human eye
- Personalized Treatment Planning: by integrating genomics, pathology, and clinical data, AI will help tailor therapies to each patient’s unique tumor biology and immune microenvironment
- Predicting Treatment Response: AI-driven models will forecast which patients are most likely to benefit from immunotherapy, chemotherapy, or targeted therapies, reducing trial-and-error approaches
- Real-Time Surgical & Clinical Decision Support: Intraoperative AI-guided pathology and imaging tools will assist surgeons in defining tumor margins and adapting treatments during procedures
- Drug Discovery & Clinical Trials: AI will accelerate discovery of new drug targets, optimize trial design, and identify biomarker-enriched patient populations to speed translation from lab to clinic
The future of AI in HNC will mean discriminating different types of HNC, guiding therapy (whether to escalate or de-escalate), predicting side effects, and deciphering cancer biology. AI enables smarter, more tailored care, but success depends on data quality, ethical design, and integration into clinical workflows.
With the help of AI, more personalized methods will be developed. It will also help researchers understand cancer biology, enabling them to identify new targets and go after the next generation of treatment for cancer patients. “If too complex, this will not be feasible to be done routinely in clinics,” Dr. Chinn said. “It is an area we have to really think about.”